Describe One Way to Differentiate Purines From Pyrimidines
The 5 nitrogenous bases. Pick more than one Pyrimidines consist of a one-ring structure.
Difference Between Purine Pyrimidine Youtube
Purines have two carbon-nitrogen rings and pyrimidines have one carbon-hydrogen ring.

. Pryimidine bases are composed of a single ring structure whereas Purines consist of fused double ring. Class of nucleotides with one ring. However pyrimidines contain one carbon-nitrogen ring and purines contain two carbon-nitrogen rings.
Biochemistry Nucleic Acids Purine and. Purines have higher melting and boiling points than pyrimidines. Pyrimidines differ from purines by their structure and the nucleotide bases they involve.
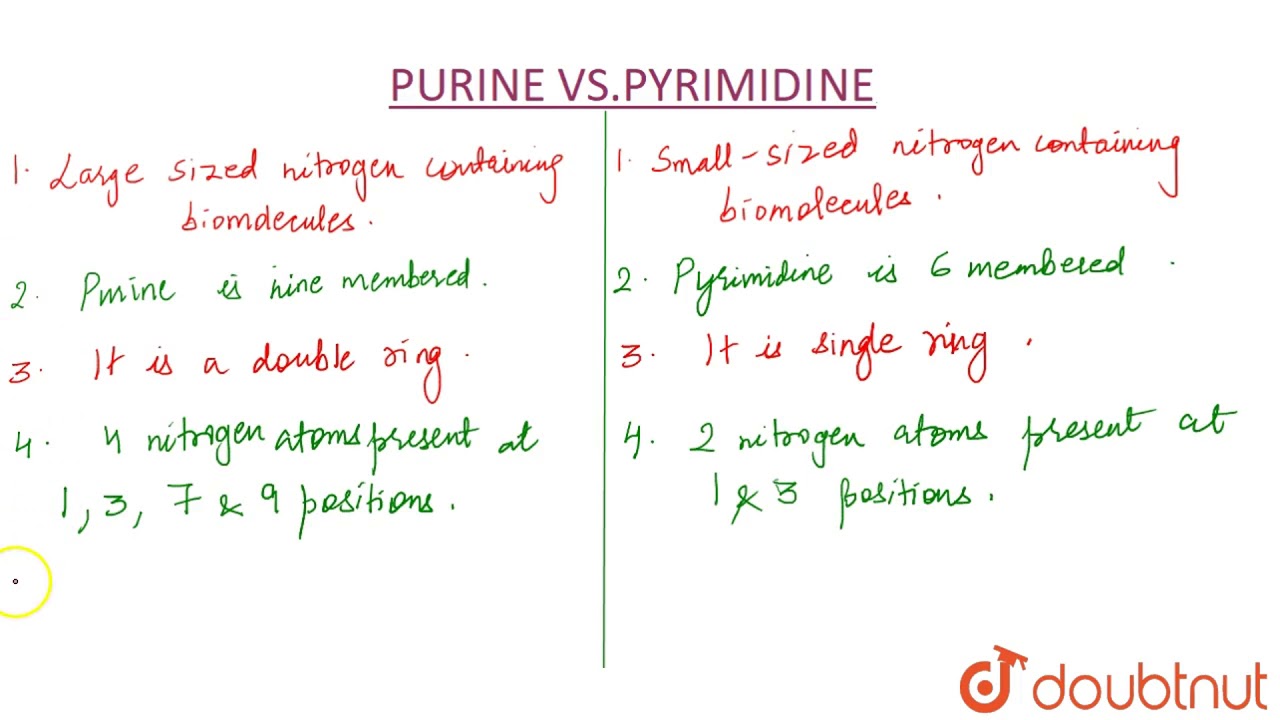
The main difference between purines and pyrimidines is that purines contain a six-membered nitrogen-containing ring fused to an imidazole ring whereas pyrimidines contain only a six-membered nitrogen-containing ring. The purines molar mass is 12011 g mol-1 and for pyrimidines the molar mass is 80088 g mol-1. Pyrimidine is a heterocyclic aromatic organic compound composed of nitrogen and carbon.
Lets begin on what they have in common then look at differences. De-novo synthesis of Pyrimidines Uracil Thymine Cytosine Biosynthesis of pyrimidines is simple than that of purines. Unlike purine synthesis pyrimidines are synthesized as bases and latter it is added to ribose sugar ie the ring is completed before being it.
The reason behind this difference in melting and boiling points is that the molecules of purines are complex and heavy. Amino Acids Given below in a tabular column are the differences between Purines and Pyrimidines. Purines are constructed using nine-member molecules with four nitrogen atoms whereas pyrimidines are constructed using just 6 members molecules and two nitrogen atoms.
So the purines are gonna have to rings in their structure. The very major and prominent difference between purines and pyrimidines is that purines are di-ring while pyrimidines are mono-ring compounds which mean purines are made of 2 rings attached while pyrimidines are made of a single ring. In Purines the purine ring is built on the sugar.
YOU MIGHT ALSO LIKE. Purine is also a heterocyclic aromatic organic compound composed of a pyrimidine ring fused to an imidazole ring. Pyrimidines form hydrogen bonds with purines.
PLTW PBS Virtual Laboratory Notebook 115 DNA Evidence Record how bases pair with each other. Should I describe the differences of their formulas. Better have a look at the most simple way to.
Purines adenine and guanine are two-carbon nitrogen ring bases while pyrimidines cytosine and thymine are one-carbon nitrogen ring bases. The three sort of nucleotide bases that are derivatives of pyrimidines includes Uracil Thymine and Cytosine. Another nice one - PS yet again.
The purines have a melting point of 214 C 487K and the pyrimidines have a melting point of 20-22C room temperature. When you think about the pyrimidines because theyre sharp and they cut theyve been cut so they only have one ring. The major difference between purines and pyrimidines is their structure.
These are organic compounds that are a part of the nucleic acids DNA RNA synthesis. In pyrimidines the pyrimidine ring is assembled without the sugar. Pyrimidines form covalent bonds with purines.
Both are nitrogenous bases. Uracil thymine cytosine and orotic acid. Unlike the purines pyrimidines have a single carbon-nitrogen ring that is attached or linked with the two nitrogen atoms.
To differentiate their bases Pyrimidines have a six-member nitrogen-containing ring while purine consists of five-membered plus six-membered nitrogen-containing rings that are stuck together. Difference between Purine and Pyrimidine. Figure 6-25 gives a general diagram recapitulating the main steps of the biosynthesis of precursors of ribonucleic acids purine and pyrimidine ribonucleosides-5-triphosphates and deoxyribonucleic acids purine and pyrimidine deoxyribonucleosides-5 triphosphates.
If all life has DNA and DNA. What conclusions can you draw about how purines and pyrimidines pair. Examples of purines are.
Adenine and guanine are purines while thymine cytosine and uracil are pyrimidines. In case of purines the carbon-rings are two in number. XD mnemonic to easily tell apart purine and pyrimidine from a given structure only purine from pyrimidine not cytosine from uracil or adenine from guanine-pyrimidine- long name short structure 1 ring purine.
The core difference between purines and pyrimidines is that purines can be created artificially by Traube purine synthesis while pyrimidine can be created artificially by Biginelli Reaction. One can have for example. Both are heterocyclic aromatic organic compounds and carry nitrogen atoms.
Purines are bigger in size than pyrimidines as the former is a two ringed structure as opposed to a structure with one ring. Purines participate in greater number of molecular reactions in comparison to pyrimidines. Short name long structure 2 rings Reply Delete.
Describe one way to differentiate purines from pyrimidines. Which of the statements describes purines and pyrimidines in DNA molecules. Adenine guanine hypoxanthine and xanthine while examples of pyrimidines are.
So thats an extra bonus to help you remember that purines have two rings and pyrimidines only have one ring. Adenine and guanine are pyrimidines. Purines are quite common in meat products such as liver and kidney.
Purines form hydrogen bonds with purines.
Difference Between Purines And Pyrimidines Definition Structure Properties


Comments
Post a Comment